Early in the development of mRNA vaccine technology fetal cells were used for proof of concept to demonstrate how a cell could take up mRNA and produce the SARS-CoV-2 spike. Elasomeran codenamed mRNA-1273 and sold under the brand name Spikevax is a COVID-19 vaccine developed by Moderna the United States National Institute of Allergy and Infectious Diseases NIAID and the Biomedical Advanced Research and Development Authority BARDA.

The Covid 19 Vaccine Communication Handbook A Practical Guide For Improving Vaccine Communication And Fighting Misinformation Tool Detail Rri Tools
It took only 10 years from the discovery of the pathogen to the development of the first vaccine.

Covid vaccine wiki. The available data on the COVID-19 vaccine BIBP in pregnant women are insufficient to assess either vaccine efficacy or vaccine-associated risks in pregnancy. Two mRNA vaccines one by Pfizer and one by Moderna were approved late in 2020. The measles vaccine was found relatively rapidly.
The COVID-19 vaccination campaign in the United States is an ongoing mass immunization campaign for the COVID-19 pandemic in the United StatesThe FDA granted emergency use authorization to the PfizerBioNTech vaccine on December 10 2020. The mRNA COVID-19 vaccines produced by Pfizer and Moderna do not require the use of any fetal cell cultures in order to manufacture produce the vaccine. To trigger an immune response many vaccines put a weakened or inactivated germ into our bodies.
In another study which was published from West Bengal a state in Eastern India has shown that 7727 of people want to take the COVID-19 vaccine. Modernas COVID-19 vaccine also known as mRNA-1273 is a two-dose vaccine. The Moderna COVID19 vaccine pINN.
New Approach to Vaccines mRNA vaccines are a new type of vaccine to protect against infectious diseases. COVAX is co-led by the Coalition for Epidemic Preparedness Innovations CEPI Gavi and WHO with UNICEF as a key delivery partner and PAHO as the procurement agent in the Americas. The known and potential benefits of COVID-19 vaccination outweigh the known and potential risks including the possible risk of myocarditis or pericarditis.
From Simple English Wikipedia the free encyclopedia A COVID-19 vaccine is any of the vaccines used against the Coronavirus Disease 2019 virus caused by SARS-CoV-2. It is authorized for use in people aged 12 years and older in some jurisdictions. The Moderna vaccine was granted emergency use authorization on December 17 2020 and the Janssen.
The COVID-19 vaccination program in Canada is an ongoing intergovernmental effort coordinated between the bodies responsible in the Government of Canada to acquire and distribute vaccines to individual provincial and territorial governments who in turn administer approved COVID-19 vaccines during the COVID-19 pandemic in CanadaSome provinces have asked local municipal governments. Messenger RNA vaccinesalso called mRNA vaccinesare some of the first COVID-19 vaccines authorized for use in the United States. COVID-19 qarşı peyvənd.
COVAX aims to accelerate the development and manufacturing of COVID-19 vaccines and guarantee fair and equitable access for every country. In July 2020 more than 150 vaccines were being developed in different laboratories. Vaccine acceptance in India.
CDC has received increased reports of myocarditis and pericarditis in adolescents and young adults after COVID-19 vaccination. 206 rows Health officials distribute the Moderna COVID-19 vaccine to frontline health workers and. The speed at which the first COVID-19 vaccines were developed was extraordinary.
One study published on the vaccine acceptance is showing that 795 of people from New Delhi a state in Northern India want to take a COVID-19 vaccine. The doses are administered 28 days apart and the vaccine trains the immune system to. We have previously looked into the history of vaccine development.
Mass vaccinations began on December 14 2020. However this vaccine is an inactivated vaccine with an adjuvant that is routinely used in many other vaccines with a documented good safety profile including in pregnant women.

Covid 19 Vaccination In South Africa Wikipedia

Vaksin Rna Wikipedia Bahasa Indonesia Ensiklopedia Bebas
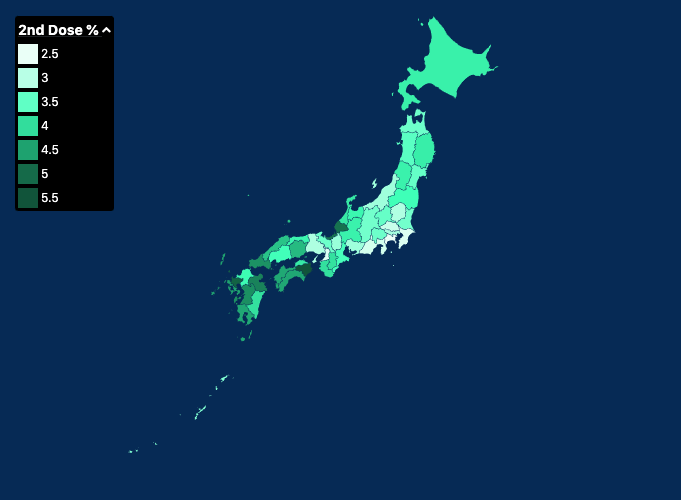
Covid 19 Vaccination In Japan Wikipedia

Sputnik V Covid 19 Vaccine Wikiwand

Covid 19 Vaccination In France Wikipedia

Vaksin Covid 19 Johnson Johnson Wikipedia Bahasa Indonesia Ensiklopedia Bebas

Covid 19 Vaccine Certification A Contentious Proposal Impact Ethics
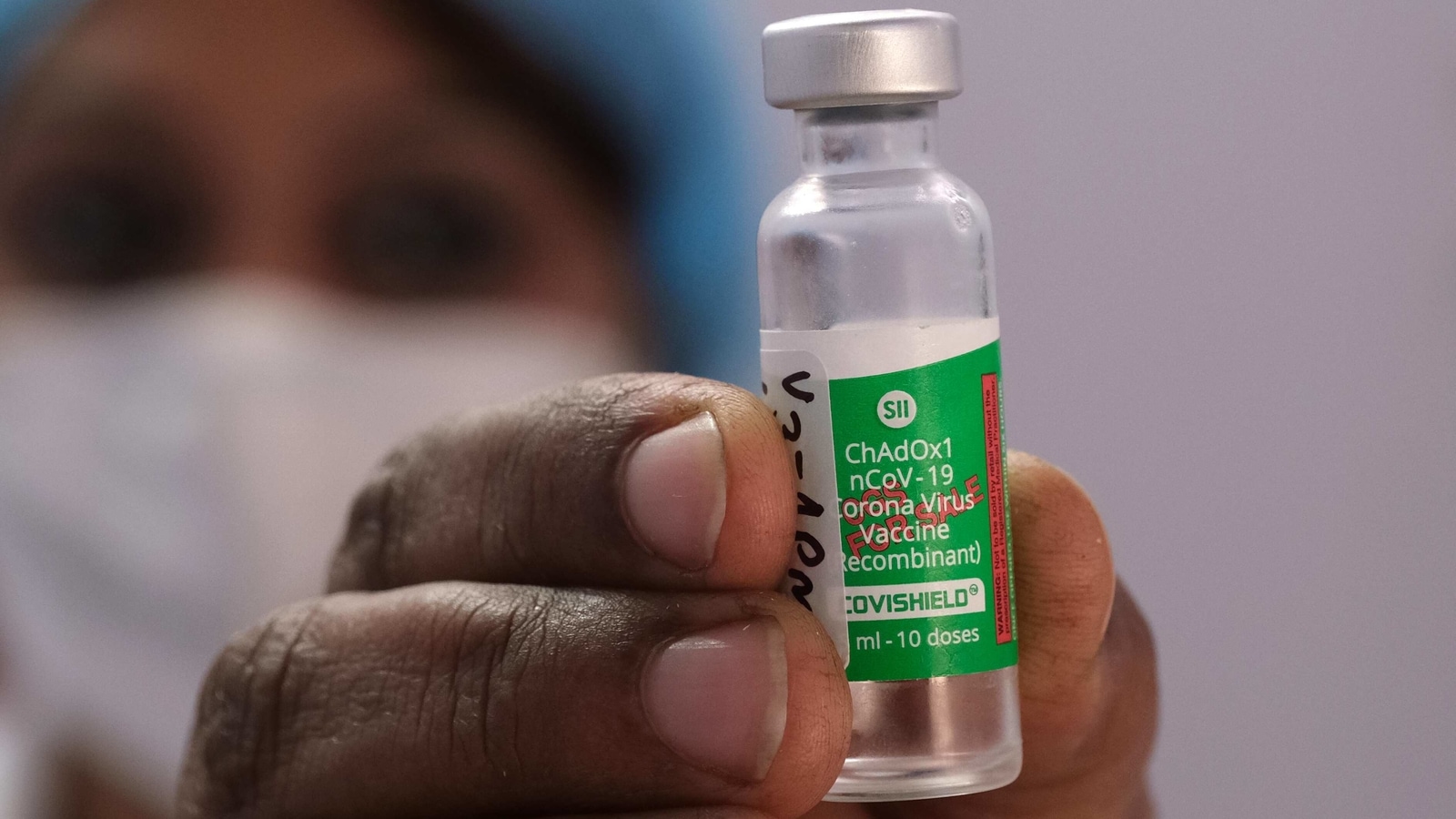
Covishield A Timeline Of The Covid 19 Vaccine Manufactured By Serum Institute Of India Latest News India Hindustan Times
How The U S Government Bolstered Moderna S Covid 19 Vaccine Candidate Drug Discovery And Development

Covid 19 Vaccination In Malaysia Wikipedia

Moderna Covid 19 Vaccine Wikiwand
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Covid 19 Vaccination Clinic Coming To Harrison Eagle Country 99 3